Non inferiority studies
Non-Inferiority and Superiority Trials
The objective of non-inferiority trials is to compare a novel treatment to an active treatment with a view of demonstrating that it is not clinically worse with regards to a specified endpoint. It is assumed that the comparator treatment has been established to have a significant clinical effect (against placebo).
These trials are frequently used in situations where use of a superiority trial against a placebo control may be considered unethical.
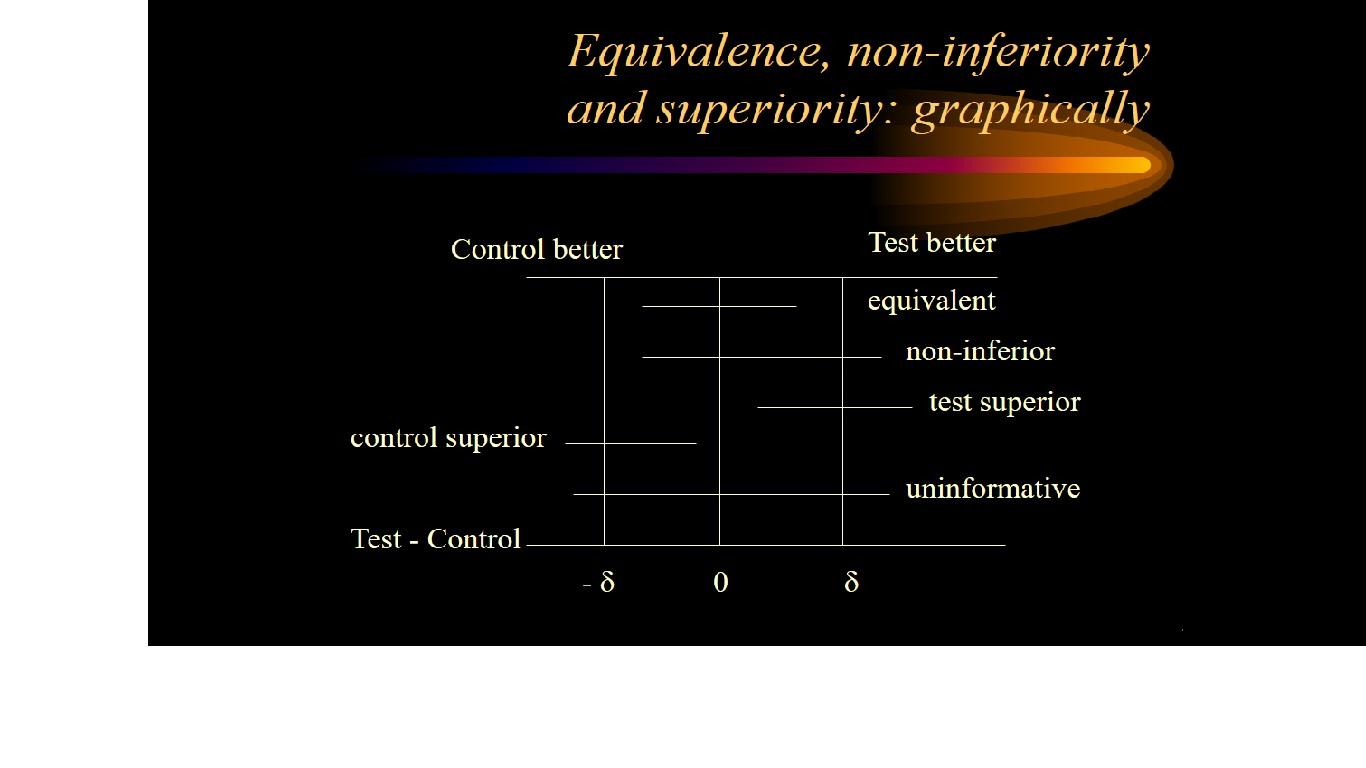
Non-inferiority is most easily assessed using a confidence interval (CI) approach.
Firstly the trial investigators specify a non-inferiority margin, delta. This is the maximum difference that they are prepared to tolerate in a given direction if the new treatment is not to be considered (clinically) inferior.
If a 95% confidence interval for the difference between treatment means lies above or below this boundary value (in a favourable direction) then non-inferiority is deemed to have been established.
Logic of Non-Inferiority studies
- If a standard S is consistently superior to placebo, then
- to show that a test treatment T is superior to placebo
- it suffices to show that the test treatment is as good as (not inferior) to the standard
Setting the non-inferiority margin
- subjective - often contentious
- if too large:
- inferior treatments may be called non-inferior
- if too small: huge sample sizes are required
- usually a fraction of the historical difference between control and placebo
Interpreting a noninferiority trial as a superiority trial
Reference:
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.