Testosterone supplementation in older men
Treating testosterone deficiency
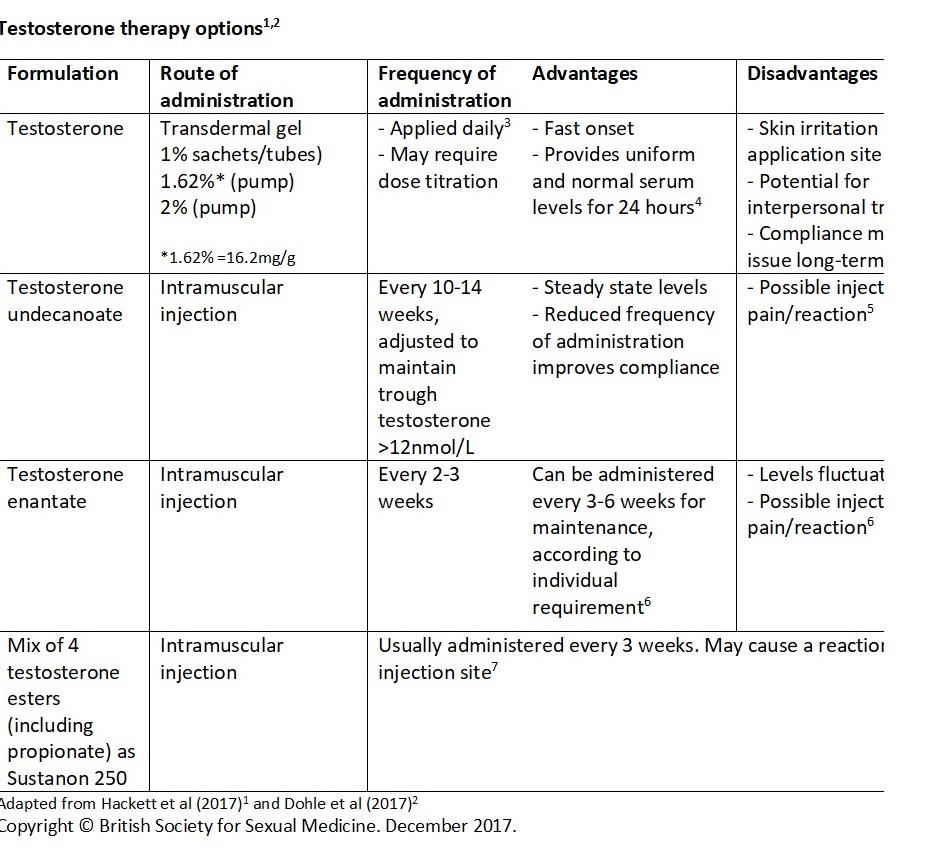
The patient should be fully informed about the expected benefits and side effects of testosterone therapy (T therapy), to facilitate a joint decision on treatment choice.
Physicians should also consider drug withdrawal times, based on the pharmacodynamic and pharmacokinetic properties of the injectable versus the transdermal formulations.
References
- 1. Hackett G, Kirby M, Edwards D, et al. UK policy statements on testosterone deficiency. Int J Clin Pract 2017;71.
- 2. Dohle GH et al. EAU guidelines on male hypogonadism (Limited text update March 2017). Available at: http://uroweb.org/guideline/male-hypogonadism/?type=pocket-guidelines (Accessed September 2017).
- 3. Besins Healthcare (UK) Ltd. Testogel Summary of Product Characteristics. May 2016.
- 4. Dohle GH, Arver S, Bettochi C et al. Guidelines on Male Hypogonadism. European Association of Urology 2017. Available at: http://uroweb.org/guideline/male-hypogonadism/ (Accessed May 2018).
- 5. Bayer PLC. Nebido 1000mg/4ml, solution for injection Summary of Product Characteristics. August 2017
- 6. Alliance Pharmaceuticals. Testosterone enantate ampoules Summary of Product Characteristics. October 2016. 7. Aspen Pharma Trading Limited. Sustanon 250 Summary of Product Characteristics. December 2016.
Contraindications to testosterone therapy
The main contraindications to testosterone therapy (T therapy) are:1
- Prostate cancer (locally advanced or metastatic)
- Male breast cancer
- An active desire to father children
- Haematocrit >54%
- Severe chronic heart failure (NYHA class IV)
An unevaluated prostate nodule or induration, or raised prostate specific antigen (PSA), should be fully investigated prior to starting T therapy.
Untreated sleep apnoea and severe LUTS have previously been considered contraindications to T Therapy, but more recent evidence suggests this may no longer be the case.2 These conditions should however, be fully investigated and optimally manged before starting treatment with testosterone.
References
- 1. Dohle GH, Arver S, Bettochi C et al. Guidelines on Male Hypogonadism. European Association of Urology 2017. Available at: http://uroweb.org/guideline/male-hypogonadism/ (Accessed May 2018).
- 2. Seftel AD, Kathrins M, Niederberger C. Critical update of the 2010 Endocrine Society clinical practice guidelines for male hypogonadism: a systematic analysis. Mayo Clin Proc 2015;90:1104-1115
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.