E-cigarette use or vaping associated lung injury (EVALI)
E-cigarette use or vaping associated lung injury
In the USA it has been suggested that E-cigarette use or vaping associated lung injury (EVALI) may be related to use of e-cigarette devices to deliver chemicals, including tetrahydrocannabinol (THC) and cannabinoids, in an aerosol that contains vitamin E (1)
- however the the exact cause of EVALI remains unclear (1)
However of note is that THC is illegal in the UK under the Misuse of Drugs Act (1971). Also vitamin E, along with other vitamins, is not permitted as an ingredient in notifiable nicotine e-cigarettes or e-liquids in the UK.
MHRA have issued guidance regarding E-cigarette use or vaping associated lung injury (2):
- authorities in the USA are investigating a multistate outbreak of e-cigarette or vaping associated lung injury, also known as EVALI. The US outbreak peaked in summer 2019 and at the time of publication (28/1/2020), the outbreak in the USA seems to be in decline
- from March 2019 to January 2020, more than 2600 cases of EVALI and 60 associated deaths were reported in the USA -THC is illegal in the UK under the Misuse of Drugs Act (1971)
- where data on hospitalisation status are known, 95% of cases were hospitalised and a proportion required intensive care
- most cases presented with non-specific respiratory symptoms such as cough, dyspnoea (shortness of breath), and chest pain, often with associated gastrointestinal symptoms, fever, chills and weight loss
- radiological findings include pulmonary infiltrates on chest X-rays and bilateral ground glass infiltrates on CT thorax (3)
- CDC considers some chronic conditions, including cardiac disease, chronic respiratory disease, and diabetes, as well as increasing age, to be potential risk factors for rehospitalisation and death
- most cases (86%) reported the use of tetrahydrocannabinol (THC)
- 11% of cases report use of nicotine liquids exclusively
- vitamin E acetate, used as an additive in THC-containing vaping products, has been identified by the CDC as a chemical of concern - however, evidence is insufficient to rule out the contribution of other chemicals and there may be more than one cause
- vitamin E, along with other vitamins, is not permitted as an ingredient in notifiable nicotine e-cigarettes or e-liquids in the UK
- from March 2019 to January 2020, more than 2600 cases of EVALI and 60 associated deaths were reported in the USA -THC is illegal in the UK under the Misuse of Drugs Act (1971)
UK context and cases
- estimated that 3.6 million people use e-cigarettes in the UK
- nicotine-containing e-cigarettes and e-liquids have been regulated since the introduction of the Tobacco and Related Products Regulations (TRPR) in 2016
- as of 8 January 2020, there were 244 suspected adverse reaction reports (182 relating to respiratory terms) to the MHRA's Yellow Card Scheme and received via industry associated with e-cigarettes or e-liquids
- of these, 20 reports describe 27 serious respiratory events including lipoid pneumonia, hypersensitivity pneumonitis, pulmonary fibrosis, pleural effusion, pneumothorax, lower respiratory tract infection, and infectious pneumonia
- four Yellow Card reports included a fatal outcome
- however, not all are considered to be causally associated
- four Yellow Card reports included a fatal outcome
- of these, 20 reports describe 27 serious respiratory events including lipoid pneumonia, hypersensitivity pneumonitis, pulmonary fibrosis, pleural effusion, pneumothorax, lower respiratory tract infection, and infectious pneumonia
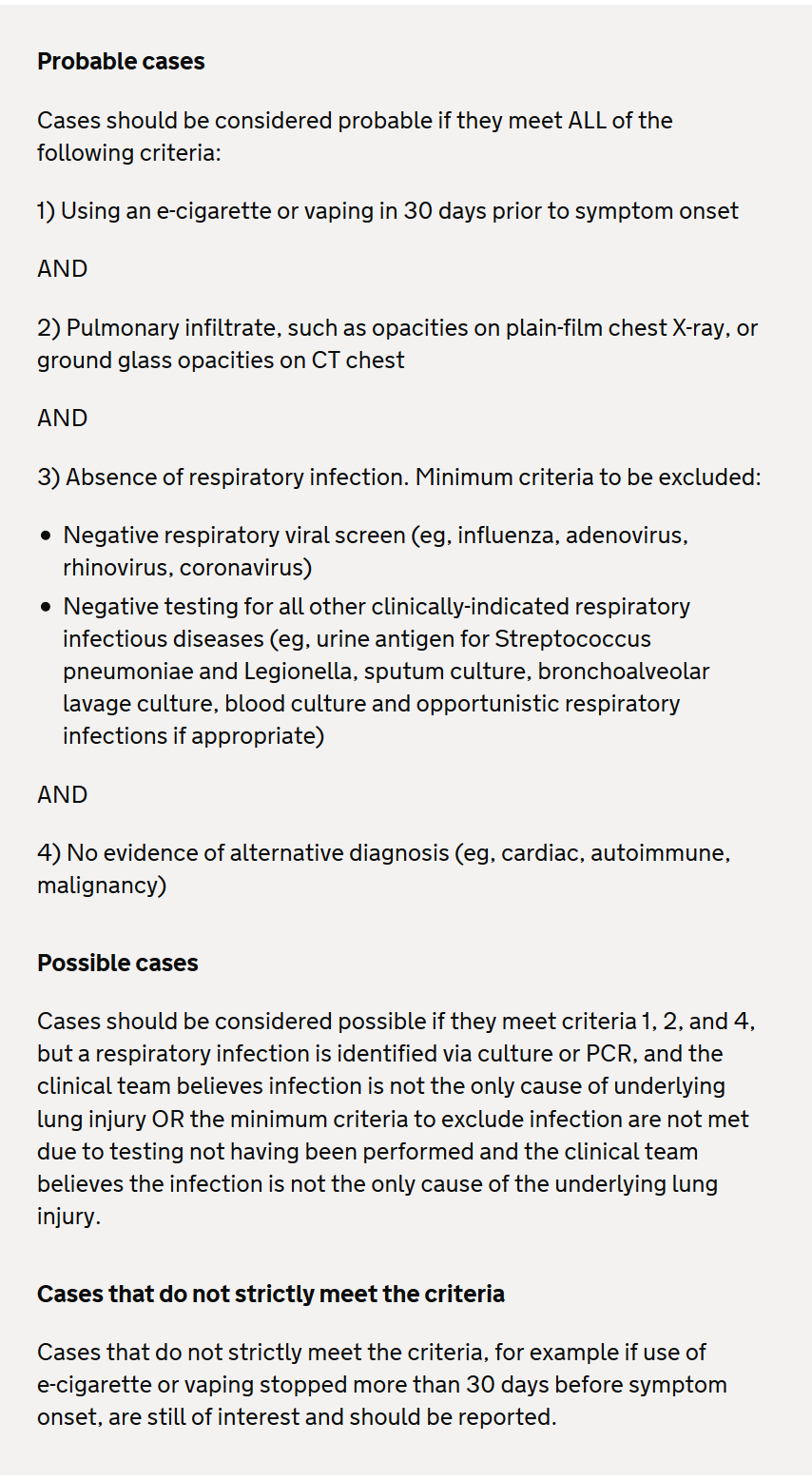
Case definitions of e-cigarette or vaping associated lung injury
The MHRA have devised UK case definitions definitions of e-cigarette or vaping associated lung injury to facilitate identification.

Actions needed from healthcare professionals:
- have a high index of suspicion in patients presenting with respiratory symptoms where there is a history of e-cigarette use or vaping in the past 30 days
- use the Yellow Card Scheme website to report any suspected side effects or safety concerns with e-cigarettes and the e-liquids used for vaping
- for all patients, ask about e-cigarette use or vaping routinely as you would do about cigarette smoking
A review suggests with respect to acquiring a vaping history (4):
- be empathetic:
- young adults may be reluctant to share history of vaping use. Familiarity with vaping terminology, asking in a non-judgmental manner, and asking in a confidential space may help
- enquire about what vape products and where the patients sources them from:
- vape products - vape pens commonly contain nicotine or an alternative active ingredient, such as THC (tetrahydrocannabinol) or CBD (cannabidiol)
- may also inquire about flavorants, or other vape solution additives, that their patient is consuming, particularly if vaping related lung injury is suspected
- source
- ask where they source their product from. Sources may include commercially available products, third party distributors, or friends or local contacts.
- vape products - vape pens commonly contain nicotine or an alternative active ingredient, such as THC (tetrahydrocannabinol) or CBD (cannabidiol)
- enquire about details of vaping:
- device
- what style of device are they using?
- frequency
- how many times a day do they use their vape pen (with frequent use considered >5 times a day)? Alternatively, providers may inquire how long it takes to deplete a vape solution cartridge (with use of one or more pods a day considered heavy use)
- nicotine concentration
- for individuals consuming nicotine-containing products, clinicians may inquire about concentration and frequency of use, as this may allow for development of a nicotine replacement therapy plan
- device
- is the patient using other inhaled produced in his/her vaping device?
- clinicians should ask patients who vape about use of other inhaled products, particularly cigarettes. Further, clinicians may ask about use of water pipes, heat-not-burn devices, THC-containing products, or dabbing
- concurrent smoking
- simultaneous use of multiple inhaled products is common among vape users, including concurrent use of conventional cigarettes, water pipes, heat-not-burn devices, and THC-containing or CBD-containing products (6)
- among those using marijuana products, gathering a history regarding the type of product use, the device, and the modality of aerosol generation may be warranted
- "dabbing" is the practice of inhaling heated butane hash oil, a concentrated THC wax - which may also be associated with lung injury
- simultaneous use of multiple inhaled products is common among vape users, including concurrent use of conventional cigarettes, water pipes, heat-not-burn devices, and THC-containing or CBD-containing products (6)
- concurrent smoking
- clinicians should ask patients who vape about use of other inhaled products, particularly cigarettes. Further, clinicians may ask about use of water pipes, heat-not-burn devices, THC-containing products, or dabbing
Reference:
- Gordon T, Fine J. Cornering the Suspects in Vaping-Associated EVALI.N Engl J Med. 2020 Feb 20;382(8):755-756
- Kalininskiy A et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach.Lancet Respir Med. 2019 Dec;7(12):1017-1026
- MHRA (28/1/2020). Drug Safety Update volume 13, issue 6: January 2020: 1.
- Jonas A. Impact of vaping on respiratory health BMJ 2022; 378 :e065997 doi:10.1136/bmj-2021-065997
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.