Cervical intraepithelial neoplasia (CIN) screening
Human papillomavirus (HPV) (1)
- high risk (HR) HPV is associated with cervical intraepithelial neoplasia (CIN) and is found in 99.7%(1) of cervical cancer cases
- high risk HPV (HR-HPV), some subtypes can cause cervical cancer. In particular HPV16 and HPV18
- high risk HPV (HR-HPV), some subtypes can cause cervical cancer. In particular HPV16 and HPV18
- persistent infection with HR-HPV is a necessary but insufficient cause of cervical cancer
- persistent HR-HPV infection increases the risk of women developing cervical cancer
- transient HR-HPV infection is common
The screening program in the UK is as follows: (2)
- aim of the NHS Cervical Screening Programme (NHS CSP) is to reduce the incidence of and mortality from cervical cancer through a systematic, quality-assured population-based screening programme for people aged 24.5 to 64 who have a cervix
- individuals are invited for their first screening test at the age of 24.5 years. Individuals under this age who have symptoms, are concerned about their sexual health, or are worried about their risk of developing cervical cancer, should contact their GP or their local genito-urinary medicine (GUM) clinic
- between the ages of 24.5 to 49, individuals are offered cervical screening every 3 years
- between the ages of 50 and 64, individuals are offered cervical screening every 5 years
Primary HPV screening
- primary human papillomavirus (HPV) screening has been demonstrated within randomised controlled trials reported in 2009, 2014 and 2019 to be more sensitive than cytology to detect pre-invasive disease of the cervix
- improved sensitivity leads to a reduction in incidence of both adenocarcinomas and squamous carcinomas of the cervix compared to cytology screening alone
- the improved sensitivity of high risk HPV (hrHPV) testing and its high negative predictive value also enables longer screening intervals for individuals with normal test results and is the optimum primary screening test for vaccinated individuals. The lower specificity of hrHPV testing requires a reflex triage test to ensure colposcopy clinics are not over burdened with unnecessary referrals and individuals are not inconvenienced
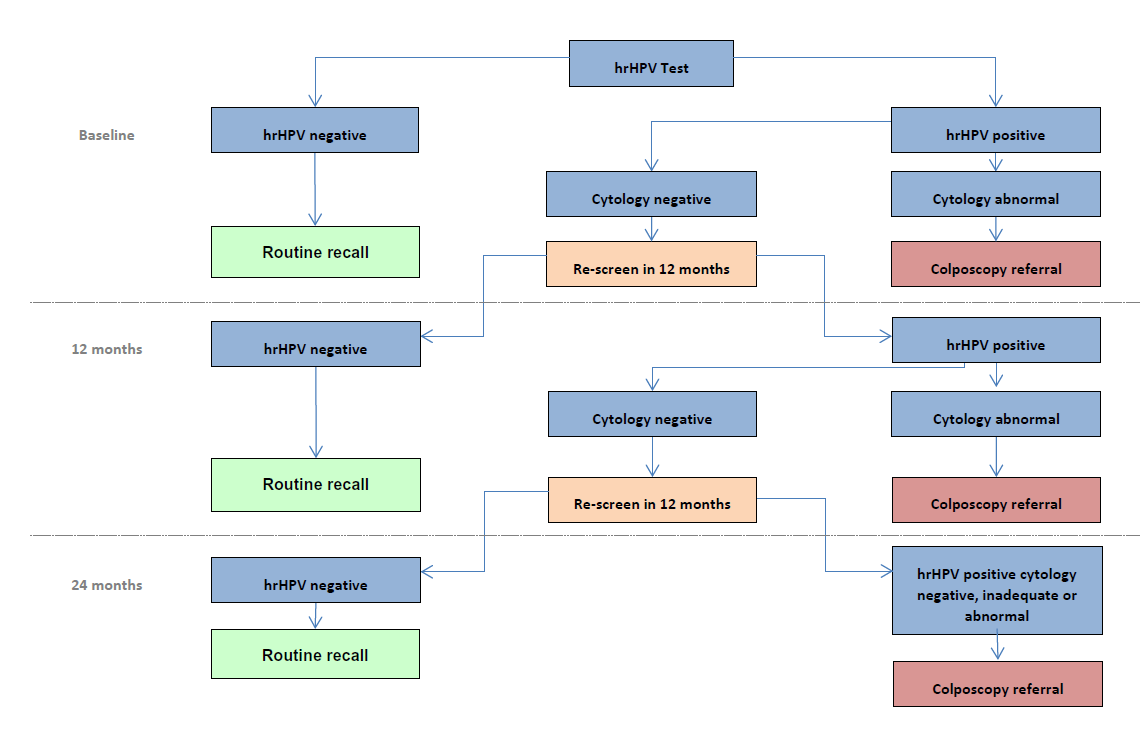
HPV in cervical screening
- high risk (HR)-HPV testing picks up more cervical abnormalities (more sensitive) than cytology, but more women without abnormalities test positive for HR-HPV (not as specific)
- women who test negative for HR-HPV have no significant cervical abnormalities (CIN2+) in 99.8% (2) of cases
- most women with high-grade abnormalities will be identified by HR-HPV testing
- as the HR-HPV test is more sensitive but less specific than cytology, primary HR-HPV testing coupled with cytology triage offers a more appropriate screening strategy, especially in an HPV-vaccinated population
Primary HR-HPV testing
- all women aged between 25 and 64 (on routine and early recall) are eligible
- the cervical sample will be taken as normal
- the sample will be tested for HR-HPV first
- samples that are positive for HR-HPV will then be processed for cytological examination (cytology triage)
- women who are HIV+ will be screened annually with the HR-HPV test in accordance with programme guidelines
Possible results
- HR-HPV not detected: return to normal recall (3 or 5 years)
- HR-HPV detected, cytology negative (no abnormal cells): recall 12 months
- HR-HPV detected, cytology positive (abnormal cells found): refer for colposcopy
- Inadequate result: repeat in 3 months
Women in follow up
- Women in follow up for treatment of CIN will be given a 3-year recall if HR-HPV negative 6 months after treatment, and will be referred to colposcopy if HR-HPV positive/any grade of cytology
- Women in follow up after adequate treatment for CGIN/SMILE will be given a 3-year recall if HR-HPV negative at both 6 and 18 months after treatment
- Women in follow up for cervical cancer (still with cervix) and CGIN/SMILE (without complete excision margins) will be screened annually with the HR-HPV test (instead of cytology) for 10 years
Unscheduled screening
Unscheduled cervical screening does not form part of the cervical screening programme. Provided an individual has undergone screening within the recommended interval (depending on their age), they should not be re-screened.
Individuals with cervical symptoms, including persistent vaginal discharge that cannot be otherwise explained (for example an infection), should be referred in a timely manner for investigation
Reference:
- Insinga RP, Liaw KL, Johnson LG, et al. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:1611-1622.
- NHS England. Cervical screening: programme overview. Published April 2025.
Related pages
- Cervical screening with HPV as primary screening test - colposcopy and management of results
- Cervical intraepithelial neoplasia
- Cervical smear
- NICE guidance - liquid based cytology for cervical screening
- Role of general practitioners in cervical screening
- Withdrawal from NHS cervical screening
- Cervical screening in under-25-year-olds
- Cervical screening in pregnancy
- Cervical screening protocol using HPV as the primary screening test
- HPV - new infection or reactivation?
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.