Retropubic mid-urethral mesh sling for stress urinary incontinence
Mid-urethral mesh sling procedure
- when considering this procedure then advise the woman that it is a permanent implant and complete removal might not be possible
- if a retropubic mid-urethral mesh sling is inserted, then the woman should be given written information about the implant, including its name, manufacturer, date of insertion, and the implanting surgeon's name and contact details
- for the procedure
- use a device manufactured from type 1 macroporous polypropylene mesh
- consider using a retropubic mid-urethral mesh sling coloured for high visibility, for ease of insertion and revision
- the procedure involves involves the passage of a small strip of tape through the retropubic space, with entry or exit points at the lower abdomen
- for the procedure
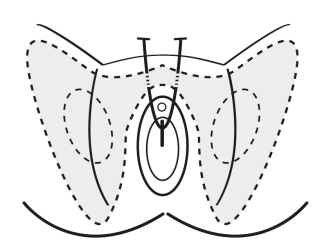
- transobturator approach should not be used unless there are specific clinical circumstances (for example, previous pelvic procedures) in which the retropubic approach should be avoided
- 'top-down' retropubic mid-urethral mesh sling approach or single-incision sub-urethral short mesh sling insertion should not be used except as part of a clinical trial
For detailed information then consult NICE guideline (1).
Reference:
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.