Antimicrobial prophylaxis in non pregnant women with recurrent urinary tract infection (UTI)
Recurrent UTI in adults is defined as repeated UTI with a frequency of 2 or more UTIs in the last 6 months or 3 or more UTIs in the last 12 months (1).
Recurrent UTI is diagnosed in children and young people under 16 years if they have (1):
- 2 or more episodes of UTI with acute pyelonephritis/upper UTI or
- 1 episode of UTI with acute pyelonephritis plus 1 or more episode of UTI with cystitis/lower UTI or
- 3 or more episodes of UTI with cystitis/lower UTI
Urinary tract infection (UTI) is defined as (1):
- typical symptoms of infection (such as dysuria, nocturia, change in urine appearance or odour) with a clinical response to antibiotics, even in the
absence of microbiological confirmation, or, - typical symptoms of infection with a positive urine dipstick (positive for nitrite or leukocyte and red blood cells), or,
- typical symptoms of infection with a positive urine culture
Urinary tract infection (UTI) is defined as (2):
- typical symptoms of infection (such as dysuria, nocturia, change in urine appearance or odour) with a clinical response to antibiotics, even in the
absence of microbiological confirmation, or, - typical symptoms of infection with a positive urine dipstick (positive for nitrite or leukocyte and red blood cells), or,
- typical symptoms of infection with a positive urine culture
Recurrent UTI >= 3 per year
Recurrent urinary tract infections (UTIs) in women are common, result in considerable morbidity and expense, and can be a management problem for clinicians. Behavioural changes can be useful antimicrobial-sparing measures in the prevention of recurren UTIs, but antimicrobial prophylaxis may be necessary in those who continue to have recurrences. Continuous prophylaxis, post-coital prophylaxis and intermittent self-treatment with antimicrobials have all been demonstrated to be effective in the prevention of recurrent uncomplicated UTIs. The decision as to which approach to use depends upon the frequency and pattern of recurrences and willingness of the patient to commit to a specific regimen.
Before any prophylaxis regime is initiated then eradication of a previous UTI should be assured by a negative urine culture one to two weeks after treatment (3,4,5)
Continuous prophylaxis:
Continuous prophylaxis has been demonstrated in numerous studies in different populations to decrease recurrences by 95% when compared with placebo or with patients' prior experience (from 2.0-3.0 episodes per patient-year to 0.1-0.2 per patient-year)
Initial trial of 6-month prophylaxis antibiotics
Most authorities advocate a 6-month trial of prophylaxis, with the dose administered at night, after which the regimen is discontinued and the patient observed for further infection. The rationale for the 6-month prophylaxis period is empiric, based on observations that UTIs seem to cluster in some women.
- before any long term prophylaxis regimen is initiated, eradication of a previous uropathogen should be confirmed by a negative urine culture 1-2 weeks after treatment
- trimethoprim 100 mg once daily OR Nitrofurantoin 50-100 mg once daily may be used (1)
- either should be tried for 6 months then stopped
- N.B. Nitrofurantoin is contraindicated if eGFR <60ml/min (due to the drug being ineffective in poor renal function as it does not concentrate in sufficient quantities in the urine)
- patients prescribed long term nitrofurantoin should be monitored closely for signs of chronic pulmonary reactions and hepatitis for full details of contraindications and side effects see the manufacturer's Summary of Product Characteristics (SPC)
- 60% of women will develop symptoms within 3-4 months of discontinuation and so will require long term prophylaxis (4,5)
- it appears that most women revert back to the earlier pattern of recurrent infections once prophylaxis is stopped unless other factors, such as sexual activity or diaphragm-spermicide use, are modified. Some authorities advocate a longer period of prophylaxis -2 or more years - in women who continue to have symptomatic infections
NICE state choice of antibiotic: people aged 16 years and over (excluding pregnant women) (1)
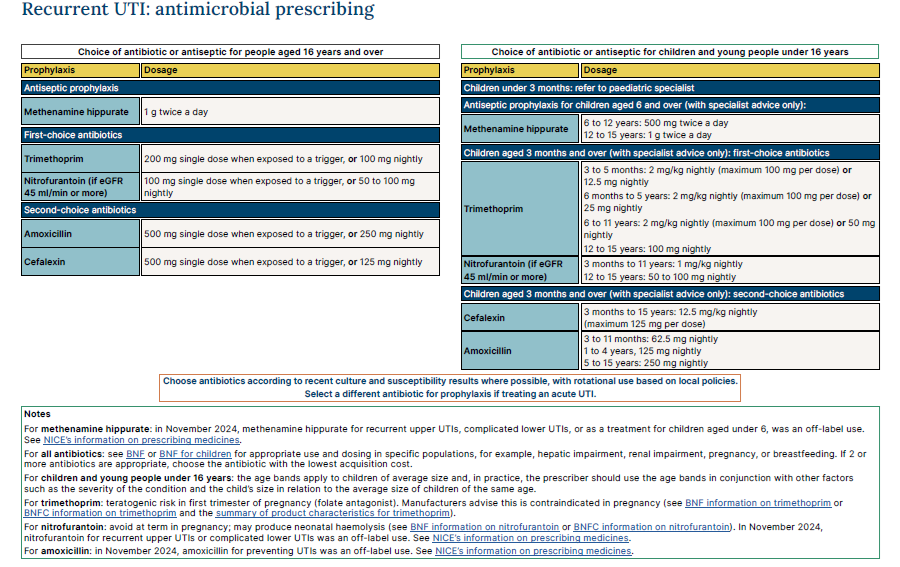
*Consult local laboratory guidance for local antibiotic policies
Reference:
- NICE (December 2024). Urinary tract infection (recurrent): antimicrobial prescribing
- Academy of Medical Royal Colleges on behalf of the Evidence-based Interventions Programme Board (January 2024). Evidence-based Interventions Clinical guidance Release 4 - Urology
- Lichtenberger P, Hooton TM. Antimicrobial prophylaxis in women with recurrent urinary tract infections.Int J Antimicrob Agents. 2011;38 l:36-41.
- Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents 2001;17:259-268.
- Drug and Therapeutics Bulletin 1998; 36(4): 30-2.
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.