Antivirals in the treatment of uncomplicated flu in the community
Last reviewed dd mmm yyyy. Last edited dd mmm yyyy
Use of Antivirals in the Treatment of adults and children in community/A&E with uncomplicated influenza (1)
1. Uncomplicated influenza:
- influenza presenting with fever, coryza, generalised symptoms (headache, malaise, myalgia, arthralgia) and sometimes gastrointestinal symptoms, but without any features of complicated influenza.
2. Complicated influenza:
- influenza requiring hospital admission and/or with symptoms and signs of lower respiratory tract infection (hypoxaemia, dyspnoea, lung infiltrate), central nervous system involvement and/or a significant exacerbation of an underlying medical condition
Prescribing in primary care (1):
- GPs may only prescribe antiviral medicines for the prophylaxis and treatment of influenza under the General Medical Services (GMS) regulations when the Chief Medical Officer (CMO) has confirmed that influenza is circulating in the community. The CMO announcement is issued to the NHS through the Central Alerting System (CAS)
- GPs have the discretion to prescribe antiviral medicines for people who are not in the specified at-risk groups but who are considered to be at risk of complications if not treated with an antiviral medicine.
All patients should be advised of the symptoms of complicated influenza and told to seek medical help should their condition worsen. The following recommendations for adults refer to dosages in Box 1. For paediatric dosing seek specialist advice Previously healthy people (excluding pregnant women): No antiviral treatment, or if physician feels patient is at serious risk of developing serious complications from influenza, then oseltamivir PO.
Consider if Risk factors for complicated influenza:
- a. Neurological, hepatic, renal, pulmonary and chronic cardiac disease.
- b. Diabetes mellitus.
- c. Severe immunosuppression.
- d. Age over 65 years.
- e. Pregnancy (including up to 2 weeks post-partum).
- f. Children under 6 months of age.
- g. Morbid obesity (BMI >=40)
Antiviral medicines may be prescribed for patients in clinical at-risk groups as well as anyone at risk of severe illness and/or complications from influenza if not treated (3):
- It is important that:
- people start taking oseltamivir within 48 hours of onset of symptoms.
- for zanamivir, treatment should begin within 48 hours of onset of symptoms for adults and within 36 hours of onset of symptoms for children who are 5 years old or over
- children over 12 months and adults who are not able to swallow capsules can be prescribed oral oseltamivir suspension
- prescribing for children under 12 months: Oseltamivir is indicated in children including full term neonates who present with symptoms typical of influenza, when influenza virus is circulating in the community
- efficacy has been demonstrated when treatment is initiated within two days of first onset of symptoms
- Clinical diagnosis of influenza may be challenging given its similarity in presentation to COVID-19
- this situation complicates recommendations for antiviral use based on clinical-epidemiological evidence alone
- as such, virological testing should be increasingly considered to guide case management and outbreak response
- this situation complicates recommendations for antiviral use based on clinical-epidemiological evidence alone
Severely immunosuppressed patients:
- some influenza subtypes are associated with a greater risk of developing oseltamivir resistance; the risk of resistance is higher in people who are severely immunosuppressed who are given antivirals, and the selection of first line antivirals in severely immunosuppressed individuals should take account of the dominant circulating strain of influenza
- oseltamivir PO is the first line treatment, unless the dominant circulating strain has a higher risk for developing oseltamivir resistance, for example influenza A(H1N1)pdm09, in which case use zanamivir (inhaled (INH))
- when oseltamivir is indicated, the manufacturer recommends a longer treatment course of 75mg PO twice daily for 10 days for immunosuppressed patients
- treatment should start as soon as possible. If clinical condition does not improve, continue with Zanamivir, take a specimen for resistance testing and consider other possible causes for a failure to improve
Suspected or confirmed oseltamivir resistant influenza in a patient who requires treatment:
- Zanamivir (INH). Treatment should be started as soon as possible. Management of patients for whom zanamivir is indicated, who are unable to self-administer inhaled zanamivir: Some patients who would normally receive inhaled zanamivir are unable to use it, either due to underlying severe respiratory disease or inability to effectively self-administer the Diskhaler (R) (this includes children under 5, for whom zanamivir is unlicensed).
Patients who are severely immunosuppressed and cannot take inhaled zanamivir should receive oseltamivir PO. As they are at increased risk of developing oseltamivir resistant influenza, they should be reviewed clinically to assess response to therapy. Patients who have suspected or confirmed oseltamivir resistant infection and cannot take inhaled zanamivir should be considered for IV zanamivir; this would be outside the marketing authorisation.
BOX 1:
Dosage in adults for treatment of uncomplicated influenza: Oseltamivir 75mg PO twice daily for 5 days Zanamivir 10mg INH twice daily for 5 days **Note: dose adjustments are required for obesity, renal dysfunction ** |
Recommended oseltamivir treatment dosing in relation to renal function (adults and those aged 13 years or over):
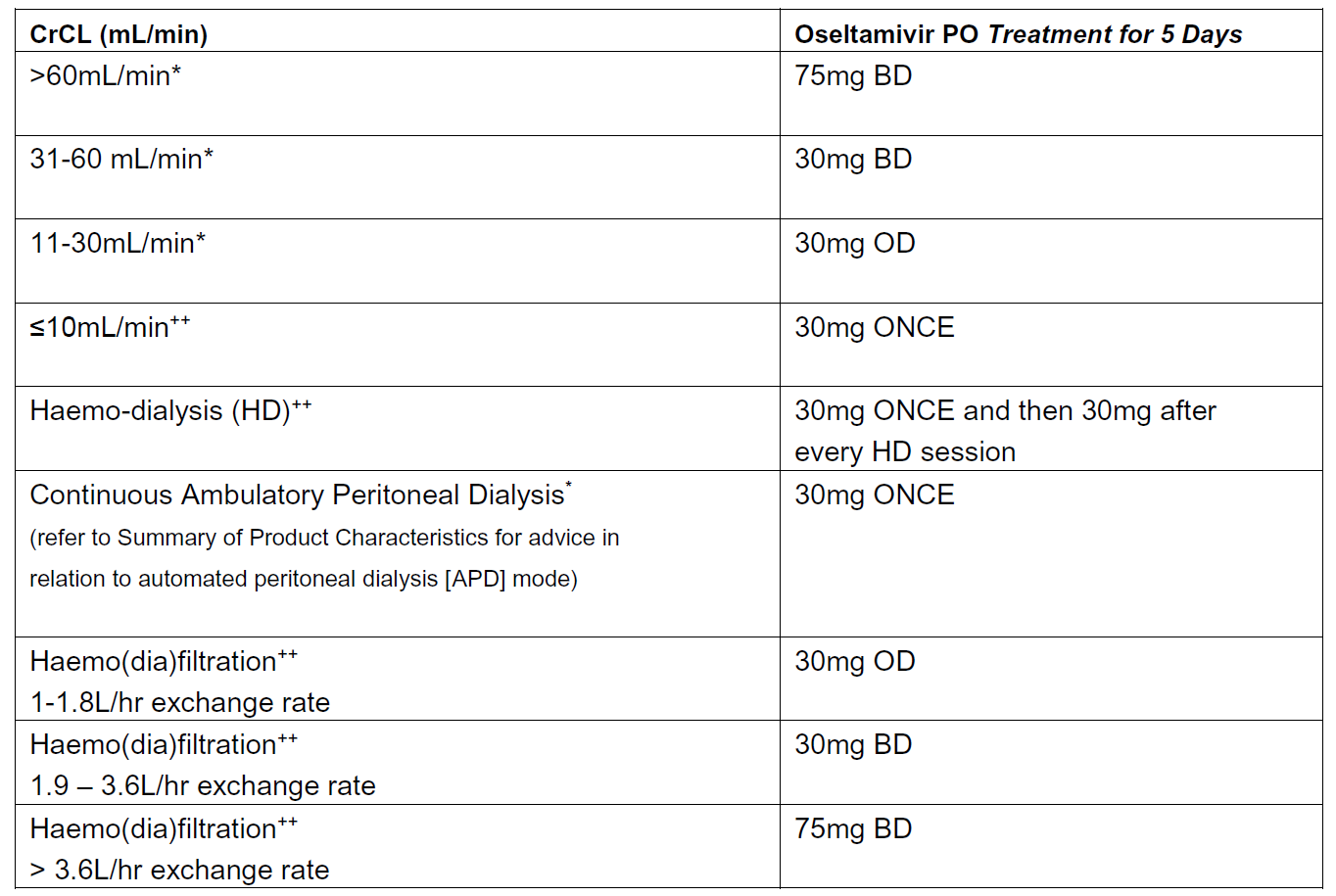
Source: Summary of Product Characteristics updated Feb 2019 (*). The recommendations for haemo-dialysis, haemo(dia)filtration and established renal failure are based on expert opinion (++)
Seek specialist advice for the use of antivirals for complicated influenza management.
Seek specialist advice for use of antivirals in children with influenza
For guidance for use of antivirals in prophylaxis of influenza then see linked item
Seek specialist advice for the use of antivirals in severe immunosuppression
Notes:
- Severe immunosuppression: Examples of severe immunosuppression relevant to this guidance are given below. Degrees of immunosuppression are difficult to quantify and individual variation exists, therefore this list is not comprehensive:
- a. Severe primary immunodeficiency
- b. Current or recent (within 6 months) chemotherapy or radiotherapy for malignancy
- c. Solid organ transplant recipients on immunosuppressive therapy
- d. Bone marrow transplant recipients currently receiving immunosuppressive treatment, or within 12 months of receiving immunosuppression
- e. Patients with current graft-versus-host disease
- f. Patients currently receiving high dose systemic corticosteroids (equivalent to >=40 mg prednisolone per day for >1 week in an adult, or >=2mg/kg/day for >=1 week in a child), and for at least 3 months after treatment has stopped.
- g. HIV infected patients with severe immunosuppression (CD4<200/µl or <15% of total lymphocytes in an adult or child over 5; CD4< 500/µl or <15% of total lymphocytes in a child aged 1 to 5; expert clinical opinion in a child aged under 1)
- h. Patients currently or recently (within 6 months) on other types of highly immunosuppressive therapy or where the patient's specialist regards them as severely immunosuppressed
Reference:
- PHE (November 2021). PHE guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza.
- NHS England (December 14th 2023). Letter from the Chief Medical Officer and Chief Pharmaceutical Officer - Influenza season 2023/24: Use of antiviral medicines.
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.