APIXABAN
Consult SPC for detailed guidance before prescribing.
Apixaban is an anti-Xa inhibitor
- available as 2.5mg and 5mg tablets.
Indications
Apixaban is indicated for
- Stroke prevention in non-valvular atrial fibrillation
- Prevention of thromboembolism post total knee replacement (TKR) and post total hip replacement (THR)
- Treatment of DVT and PE
- Prevention of recurrent DVT and PE
Contra-indications (1,2):
Active bleeding; significant risk of major bleeding (e.g. recent gastro-intestinal ulcer, oesophageal varices, recent brain, spine, or ophthalmic surgery, recent intracranial haemorrhage, malignant neoplasms, vascular aneurysm). Apixaban is not licensed for use in patients with prosthetic heart valves. Apixaban is contraindicated in severe liver disease. Apixaban should be used with caution in patients with elevated hepatic enzymes.
Initiation
- Baseline Activated Partial Prothrombin Time (aPTT), International Normalised Ratio (INR), haemoglobin, urea & electrolytes and liver function tests
- Weigh patient
- Obtain patient height
- Calculate baseline creatinine clearance (CrCl) using Cockgroft and Gault
- Informed discussion with patient regarding risks and benefits of apixaban
- Use reduced dose in patients with creatinine clearance 15-29ml/min (although some primary care guidance states to avoid because of bleeding risk (3)). Avoid use in patients with creatinine clearance <15ml/min
If patient is anticoagulated on either LMWH or a different oral agent then follow the information below:
- Parenteral anticoagulants to apixaban - Switching treatment from parenteral anticoagulants to apixaban can be done at the next scheduled dose
- Vitamin K antagonists to apixaban - When converting patients from Vitamin K antagonist (VKA) therapy to apixaban discontinue warfarin or other VKA therapy and start apixaban when the international normalized ratio (INR) is < 2.0.
Apixiban dosing advice for primary care (3):
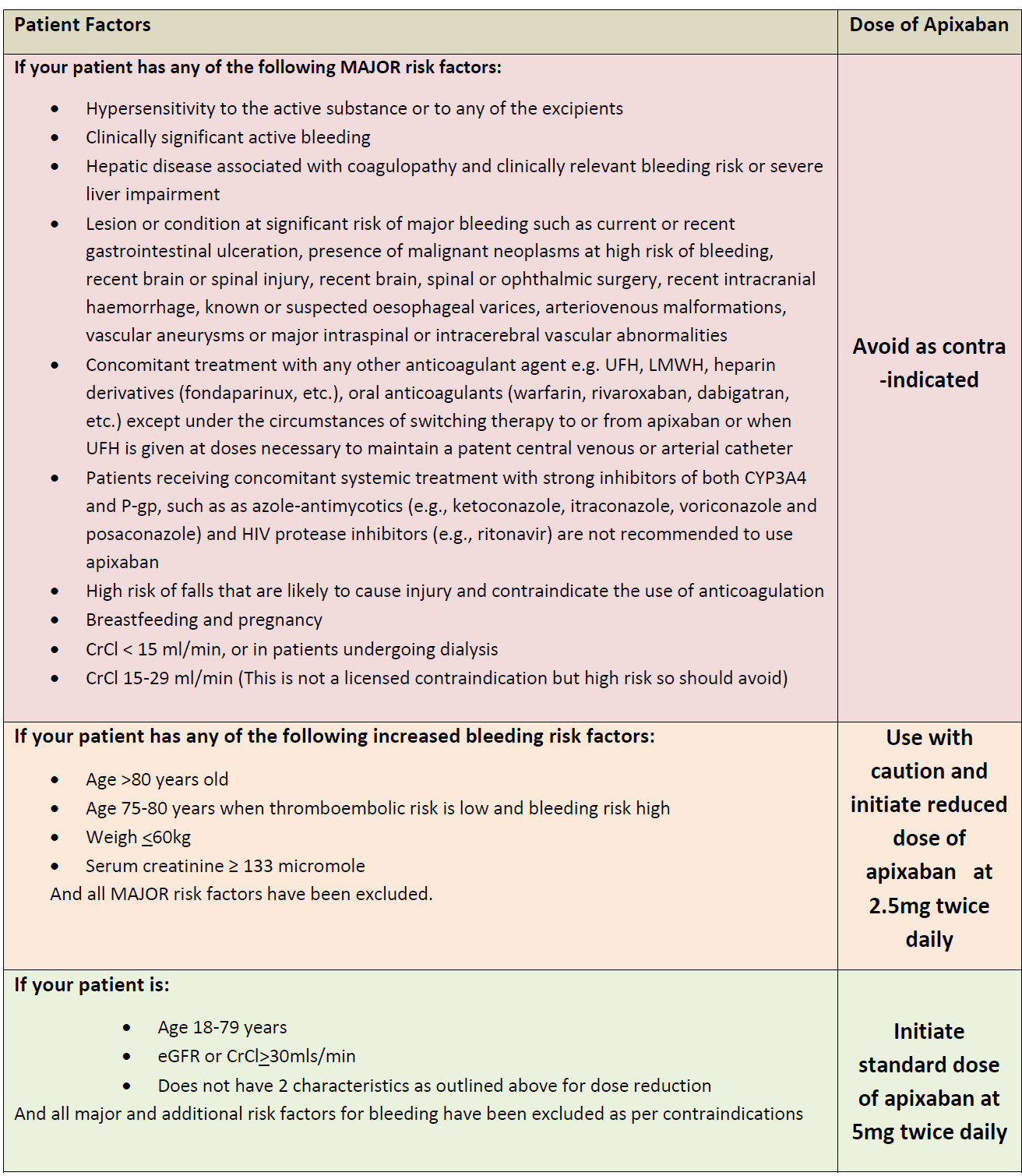
Monitoring (2)
No routine anticoagulation monitoring is needed
Patient compliance should be assessed every three months ideally.
Enquire about presence of any adverse effects, in particular signs and symptoms of bleeding and anaemia, every three months idealy
Renal function may decline whilst on treatment so it should be monitored annually if CrCl>60mL/min, every 6 months if CrCl 30-60mL/min or every 3 months if CrCl 15-30mL/min
The EHRA guidance suggests retesting every x-months (where x=CrCl/10) [e.g. if CrCl 30mL/min every 3 months, if CrCl 20mL/min every 2 months].
LFTs annually
CrCl and LFTs should be performed more often if there is an intercurrent illness that may impact renal or hepatic function
Full blood count annually
Adverse effects
Apixaban should be used with caution in conditions with an increased risk of bleeding. Bleeding may occur at any site during therapy with apixaban. An unexplained fall in haemoglobin and/or haematocrit or blood pressure should lead to an investigation to identify a bleeding site. Close clinical surveillance is recommended throughout the treatment period, especially if risk factors are combined.
Other common adverse effects include nausea, bruising, anaemia; less commonly hypotension, thrombocytopenia, rash.
Action required if abnormal results (2)
If CrCl< 15mL/min stop apixaban, assess for bleeding and seek advice regarding alternative anticoagulation therapy
If CrCl is 15-29 mL/min, the following recommendations apply:
- For prophylaxis of recurrent DVT or PE, and treatment of DVT or PE, use apixaban with caution
- For prophylaxis of stroke and systemic embolism in a person with AF, reduce the dose to 2.5 mg twice daily if CrCl is 15-29 mL/minute, or if serum creatinine is 133 micromol/litre or greater and the person is 80 years of age or older or weighs 60 kg or less
If liver enzymes are elevated (ALT/AST >2 x ULN or total bilirubin .1.5 x ULN) apixaban should be used with caution (these patients were excluded from clinical trials)
If the patient's HASBLED score is more than 3, then the patient is at a high risk of bleeding and apixaban should be used cautiously, with regular reviews
Discontinuation of therapy
If patient has reached end of duration of treatment then apixaban can be discontinued immediately.
Where switching from apixaban to alternative anticoagulant follow this guidance:
- Apixaban treatment to parenteral anticoagulant. Switching treatment to parenteral anticoagulants from apixaban can be done at the next scheduled dose
- Apixaban treatment to Vitamin K antagonists (VKA) e.g. warfarin - when converting patients from apixaban to VKA therapy, continue administration of apixaban for at least 2 days after beginning VKA therapy. After 2 days of coadministration of apixaban with VKA therapy, obtain an INR prior to the next scheduled dose of apixaban. Continue coadministration of apixaban and VKA therapy until the INR is <2.0
- If stopping apixaban for inpatients then ensure VTE assessment is redone
Reference:
- Wirral University Teaching Hospital NHS Trust. Oral Anticoagulants (VKA and DOAC) Guidelines for prescribing, monitoring and management (Accessed 23/4/19)
- NHS Specialist Pharmacy Service (October 2017). Suggestions for Drug Monitoring in Adults in Primary Care
- West Cheshire Commissioning Group (April 24th 2019). Prescribing Guidance for Apixaban.
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.