Risk groups and influenza vaccine eligibility
Influenza can affect anyone although those aged over 65 years, those with underlying health conditions, pregnant women and children under six months of age have a higher risk of developing severe disease or complications such as bronchitis or secondary bacterial pneumonia, or otitis media in children.
In the 2019/20 flu season, for those aged from six months to less than 65 years of age, clinicians should offer flu immunisation, based on individual assessment, to clinically vulnerable individuals such as those with:

Other groups
- list above is not exhaustive, and the medical practitioner should apply clinical judgment to take into account the risk of influenza exacerbating any underlying disease that a patient may have, as well as the risk of serious illness from influenza itself. Influenza vaccine should be offered in such cases even if the individual is not in the clinical risk groups specified above. Vaccination should also be offered to household contacts of immunocompromised individuals, i.e. individuals who expect to share living accommodation on most days over the winter and therefore for whom continuing close contact is unavoidable. This may include carers (see below)
- In addition to the above, immunisation should be provided to healthcare and social care workers in direct contact with patients/clients to protect them and to reduce the transmission of influenza within health and social care premises, to contribute to the protection of individuals who may have a suboptimal response to their own immunisations, and to avoid disruption to services that provide their care. This would include:
- health and social care staff directly involved in the care of their patients or clients
- those living in long-stay residential care homes or other long-stay care facilities where rapid spread is likely to follow introduction of infection and cause high morbidity and mortality (this does not include prisons, young offender institutions, university halls of residence etc.)
- those who are in receipt of a carer's allowance, or those who are the main carer of an elderly or disabled person whose welfare may be at risk if the carer falls ill. Vaccination should be given on an individual basis at the GP's discretion in the context of other clinical risk groups in their practice
- others involved directly in delivering health and social care such that they and vulnerable patiens/clients are at increased risk of exposure to influenza (further information is provided in guidance from UK health departments
Adults 65 years of age and over are a group that is recommended to have the influenza vaccine.
For vaccination of those aged 65 years and over, JCVI advises the use of any one of the following inactivated vaccines:
- adjuvanted trivalent influenza vaccine (aTIV)
- cell-based quadrivalent influenza vaccine (QIVc)
- high-dose trivalent influenza vaccine (TIV-HD)
These vaccines are considered equally suitable for use in those aged 65 years and over and are preferable to standard egg-based inactivated trivalent and quadrivalent vaccines (TIVe/QIVe). However, the high-dose trivalent influenza vaccine (TIV-HD) will not be commissioned by NHS England or reimbursed for use in the NHS Influenza vaccination programme in 2019/20 because it has a significantly higher list price
Adults (including pregnant women) aged under 65 years and children in clinical risk groups
For vaccination of adults aged less than 65 years of age, JCVI advises the use of any one of the following inactivated vaccines:
- cell-based quadrivalent influenza vaccine (QIVc)
- egg-grown quadrivalent influenza vaccine (QIVe)
These vaccines are considered equally suitable for use in those less than 65 years of age and in an at-risk group (subject to licensed age indications). These vaccines are preferable to standard egg based inactivated trivalent vaccines (TIVe).
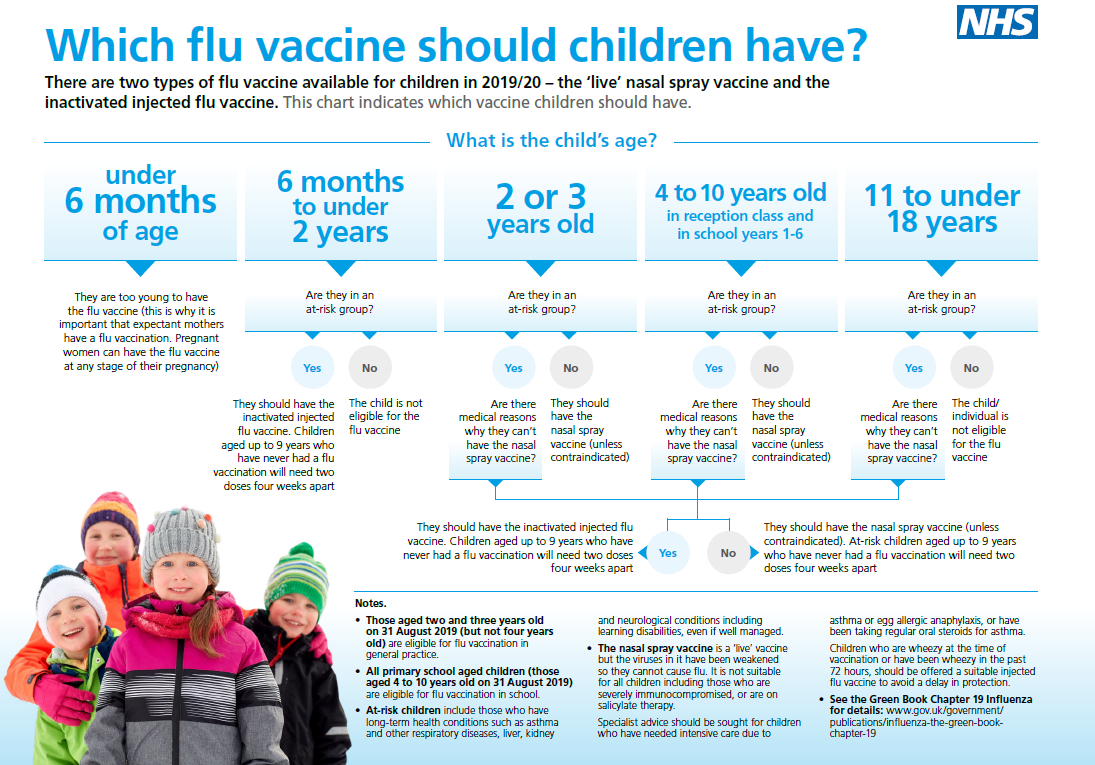
Children aged from 2 years to less than 18 years are recommended to receive the live attenuated quadrivalent influenza vaccine (LAIV), given as a nasal spray, unless contraindicated.
QIVe will be centrally supplied for children in risk groups for whom LAIV is contraindicated or otherwise unsuitable. Further information on this vaccine and the childhood flu programme can be found on the PHE national flu immunisation programme page.
As LAIV is not licenced for use in those aged 6 months to 2 years, eligible at-risk children are recommended to receive an age appropriate inactivated quadrivalent influenza vaccine (injected). QIVe will be centrally supplied for these children.
Reference:
- The Green Book. Chapter 19 - Influenza (April 2019)
- Public Health England (September 2019). The national influenza immunisation programme 2019/20 Inactivated influenza vaccine information for healthcare practitioners
- Public Health England (August 2019). A quick reference guide to Flu vaccines for children for winter 2019 to 2020.
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.