Topical HRT in the management of urogenital symptoms
Oestrogen is used for the management of urogenital symptoms (e.g. vaginal dryness, dyspareunia as a result of vaginal dryness, recurrent urinary tract infection, and urinary frequency and urgency). Alternatives for management are:
- low-dose vaginal oestrogen such as oestriol (cream, pessary or gel) and/or systemic (oral or transdermal)
- NICE states that with respect to the management of urogenital atrophy (1)
- People with no history of breast cancer
- offer vaginal oestrogen to people with genitourinary symptoms associated with menopause (including those using systemic HRT) and review regularly
- when discussing the option of vaginal oestrogen, explain that:
- serious adverse effects are very rare
- their treatment should be reviewed
- symptoms often return when vaginal oestrogen is stopped but treatment can be restarted if necessary
- vaginal oestrogen is absorbed locally – a minimal amount is absorbed into the bloodstream (when compared with systemic HRT), but this is unlikely to have a significant effect throughout the body
- when someone chooses vaginal oestrogen, make a shared decision with the person about whether to use an oestrogen cream, gel, tablet, pessary or ring
- advise people with genitourinary symptoms associated with menopause that vaginal oestrogen can be used on its own or in combination with non-hormonal moisturisers or lubricant
- for people with genitourinary symptoms in whom vaginal oestrogen preparations are contraindicated, or for people who would prefer not to use vaginal oestrogen, consider non-hormonal vaginal moisturisers or lubricants
- consider vaginal prasterone for genitourinary symptoms if vaginal oestrogen or non-hormonal moisturisers or lubricants have been ineffective or are not tolerated
- consider ospemifene as an oral treatment for genitourinary symptoms, if the use of locally applied treatments is impractical, for example, because of disability
- for the use of vaginal oestrogen in people with genitourinary symptoms and an overactive bladder, see the section on choosing medicines for an overactive bladder, in NICE's guideline on managing urinary incontinence and pelvic organ prolapse in women
- for the use of vaginal oestrogen in people with genitourinary symptoms and recurrent urinary tract infections, see the recommendations on oestrogen in NICE's guideline on recurrent urinary tract infection (UTI)
- People with a personal history of breast cancer
- offer non-hormonal moisturisers or lubricants to people with a personal history of breast cancer and genitourinary symptoms associated with menopause
- consider vaginal oestrogen for people with a personal history of breast cancer and genitourinary symptoms that have continued despite trying non-hormonal treatments
- vaginal oestrogen may be used in combination with a non-hormonal moisturiser or a lubricant
- for people currently having aromatase inhibitors as adjuvant treatment for breast cancer, work with a breast cancer specialist to identify treatment options for genitourinary symptoms that have continued despite trying non-hormonal treatments
- when assessing the safety of vaginal oestrogens for someone in relation to breast cancer recurrence, take into account all of the following:
- the person's general risk factors for breast cancer recurrence
- it is unknown whether vaginal oestrogen affects the risks of breast cancer recurrence
- vaginal oestrogen is absorbed locally, and some of it is absorbed into the bloodstream but compared with oestrogen from systemic HRT, the amount is minimal
- for people with a personal history of oestrogen receptor-negative breast cancer, recognise that any oestrogen systemically absorbed from taking vaginal oestrogen is unlikely to increase the risk of breast cancer recurrence, and so it is likely to be safe
- for people with a personal history of oestrogen receptor-positive breast cancer, recognise that:
- it is unknown whether any oestrogen systemically absorbed from taking vaginal oestrogen could increase the risk of breast cancer recurrence and
- adjuvants that block oestrogen receptors in cancer cells (for example, tamoxifen) would reduce any such potential impact
- People with no history of breast cancer
- for anyone who has been given any treatment for genitourinary symptoms associated with menopause, see the recommendations on reviewing treatment
Do not routinely offer selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs) or clonidine as first-line treatment for vasomotor symptoms alone (1).
Notes:
- improvement may take several months, and symptoms may recur if treatment is stopped
- long-term treatment is often required. Low-dose vaginal oestrogen may be preferred if the woman does not wish to take systemic HRT or cannot tolerate systemic HRT
- endometrial effects should not be incurred, and a progestogen is not needed with such low-dose preparations
- advise women using topical oestrogen therapy to contact their doctor if they experience any vaginal bleeding
- treatment should be reviewed at least annually - " systemic effects of oestrogen are minimised by using the lowest effective dose to control symptoms; the dose may be increased on the advice of a healthcare professional with expertise in menopause if there is inadequate symptom control. Treatment is continued for as long as needed to relieve symptoms and reviewed initially at 3 months, then at least annually" (2)
- if there is no symptomatic improvement with hormonal treatment, then another underlying cause of the symptoms should be considered (eg, dermatitis, vulvodynia).
Note that these products may damage latex condoms and diaphragms
Topical oestrogens - options include (2):
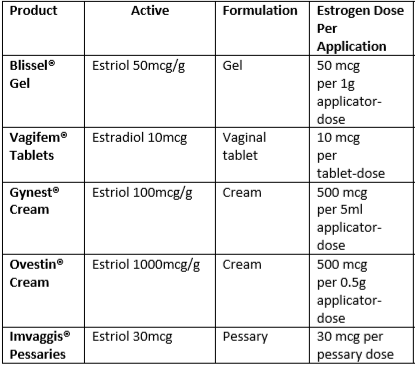
Initiating and monitoring treatment for topical oestrogens (3)
- use the lowest effective dose to minimise systemic absorption - eg, pessaries or creams or gels daily for the first two weeks and then reduce to twice weekly
- it is common to have more vaginal discharge with pessaries and creams, which may be an advantageous side effect in sexually active women
- topical vaginal oestrogen preparations reverse urogenital atrophic changes and may relieve associated urinary symptoms
- a review from the Collaborative Group on Hormonal Factors in Breast Cancer (4) “...There appeared to be little risk, however, from topical vaginal oestrogen preparations, which limit systemic exposure.”
- use of vaginal oestrogen after diagnosis of breast cancer
- a cohort study of 49,237 females with breast cancer, found that there was no evidence of an increase in early breast cancer-specific mortality with the use of vaginal oestrogen therapy compared with no hormone replacement therapy use after breast cancer diagnosis (5)
- maximum benefit with these products is usually achieved after around 1–3 months but it can take up to 1 year in some women. Treatment with topical oestrogen should be continued for as long as needed to relieve symptoms as symptoms will often return after treatment is stopped (1) - since the systemic absorption of oestrogen from recommended doses of topical oestrogens is very small (i.e. approximately 1 year's supply of topical therapy contains the same dose as taking a single tablet of oral hormone replacement therapy [HRT]), it is unlikely to be associated with the adverse effects reported with the use of systemic HRT
All NICE guidance should be viewed in close conjunction with the particular prescribing information (Summary of Product Characteristics) of individual medicinal products cited.
Reference:
- NICE. Menopause: identification and management. NICE guideline NG23. Published November 2015, last updated November 2024
- British National Formulary (BNF) (November 5th 2023)
- Al-Baghdadi O, Ewies AA; Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009 Apr;12(2):91-105
- Collaborative Group on Hormonal Factors in Breast Cancer.Type and Timing of Menopausal Hormone Therapy and Breast Cancer Risk: Individual Participant Meta-Analysis of the Worldwide Epidemiological Evidence.Lancet. 2019 Sep 28;394(10204):1159-1168.
- McVicker L, Labeit AM, Coupland CAC, et al. Vaginal Estrogen Therapy Use and Survival in Females With Breast Cancer. JAMA Oncol. Published online November 02, 2023. doi:10.1001/jamaoncol.2023.4508
*Original content created by Dr Louise Newson
Related pages
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page