Flu vaccine summary guide 2025/26
Risk groups and influenza vaccine eligibility
Eligible cohorts for flu vaccination are based on the advice of the Joint Committee on Vaccination and Immunisation (JCVI). The programme aims to provide direct protection to those who are at higher risk of flu associated morbidity and mortality and to reduce transmission to all age groups through the vaccination of children.
The following cohorts are announced and authorised to be eligible to receive a flu vaccination:
From 1 September 2025:
- pregnant women
- all children aged 2 or 3 years on 31 August 2025
- primary school aged children (from Reception to Year 6)
- secondary school aged children (from Year 7 to Year 11)
- all children in clinical risk groups aged from 6 months to less than 18 years
From October 2025:
- those aged 65 years and over
- those aged 18 years to under 65 years in clinical risk groups (as defined by the Green Book, Influenza chapter 19
- those in long-stay residential care homes
- carers in receipt of carer’s allowance, or those who are the main carer of an elderly or disabled person
- close contacts of immunocompromised individuals
- frontline workers in a social care setting without an employer led occupational health scheme including those working for a registered residential care or nursing home, registered domiciliary care providers, voluntary managed hospice providers and those that are employed by those who receive direct payments (personal budgets) or Personal Health budgets, such as Personal Assistants
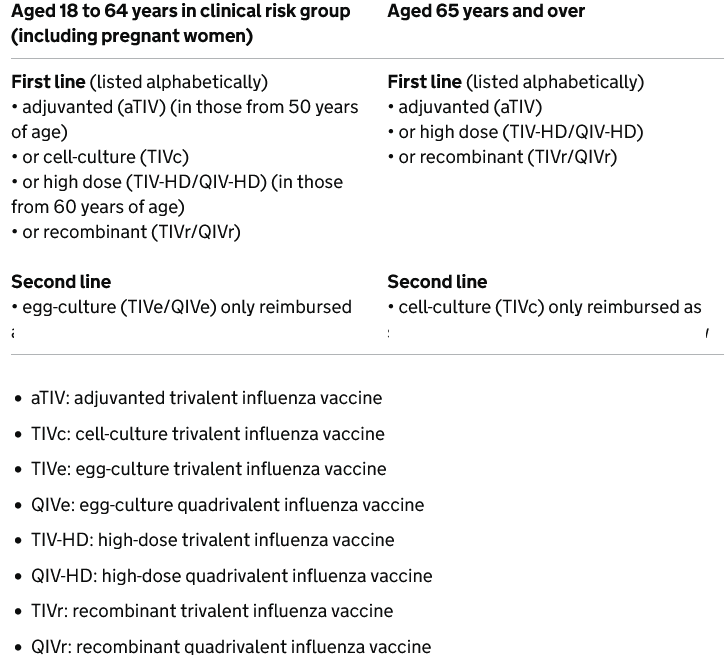
Recommended adult influenza vaccines:
Children’s vaccines
For those in clinical risk groups aged 6 months to less than 2 years
The first line vaccine is the cell-culture influenza vaccine (TIVc). Where this is not available, egg-culture influenza vaccine (TIVe/QIVe) can be offered as a second line choice.
For those aged 2 years and above in eligible and clinical risk groups
The first line choice is the live attenuated influenza vaccine (LAIV). TIVc is recommended where LAIV is contraindicated or otherwise unsuitable (for example, parents object to LAIV on the grounds of its porcine gelatine content). Where TIVc is not available, TIVe/QIVe can be offered but this is the least preferred option.
Table 2: Recommended flu vaccines for children
Children aged 6 months to less than 2 years in clinical risk groups Children aged 2 to less than 18 years in eligible groups (including clinical risk groups) Offer in the following order of preference:
First line
• TIVc
Second line
• TIVe/QIVe
Offer in the following order of preference:
First line
• LAIV
Second line
• TIVc is recommended where LAIV is contraindicated or otherwise unsuitable (for example, parents object to LAIV on the grounds of its porcine gelatine content)
Third line
• TIVe/QIVe
- TIVc: cell-culture trivalent influenza vaccine
- TIVe : egg-culture trivalent influenza vaccine
- QIVe: egg-culture quadrivalent influenza vaccine
- LAIV: live attenuated influenza vaccine
Reference:
- UK Health Security Agency (July 28th 2025). National flu immunisation programme 2025 to 2026 letter.
Related pages
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page