NICE guidance - glitazone therapy in clinical practice
Last reviewed dd mmm yyyy. Last edited dd mmm yyyy
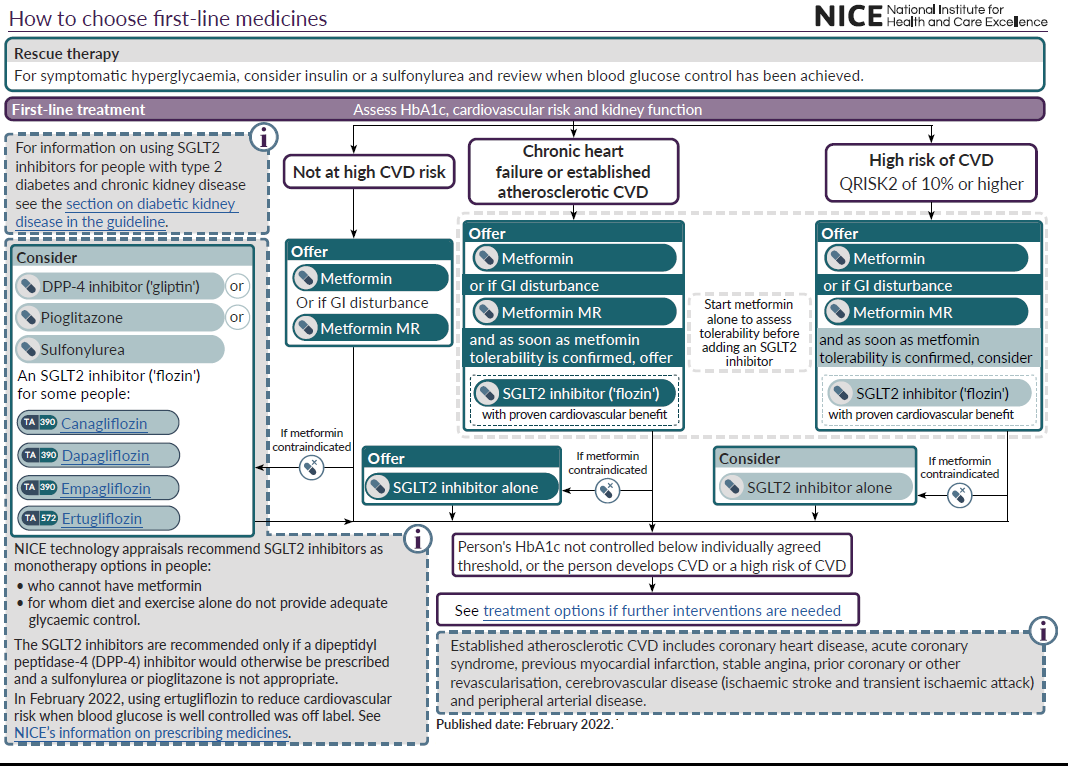
Pioglitazone should be considered for first-line drug treatment in adults with type 2 diabetes, if metformin is contraindicated or not tolerated and if they are not in either of
- have chronic heart failure or established atherosclerotic cardiovascular disease, when should offer an SGLT2 inhibitor with proven cardiovascular benefit
- are at high risk of developing cardiovascular disease, when should consider an SGLT2 inhibitor with proven cardiovascular benefit
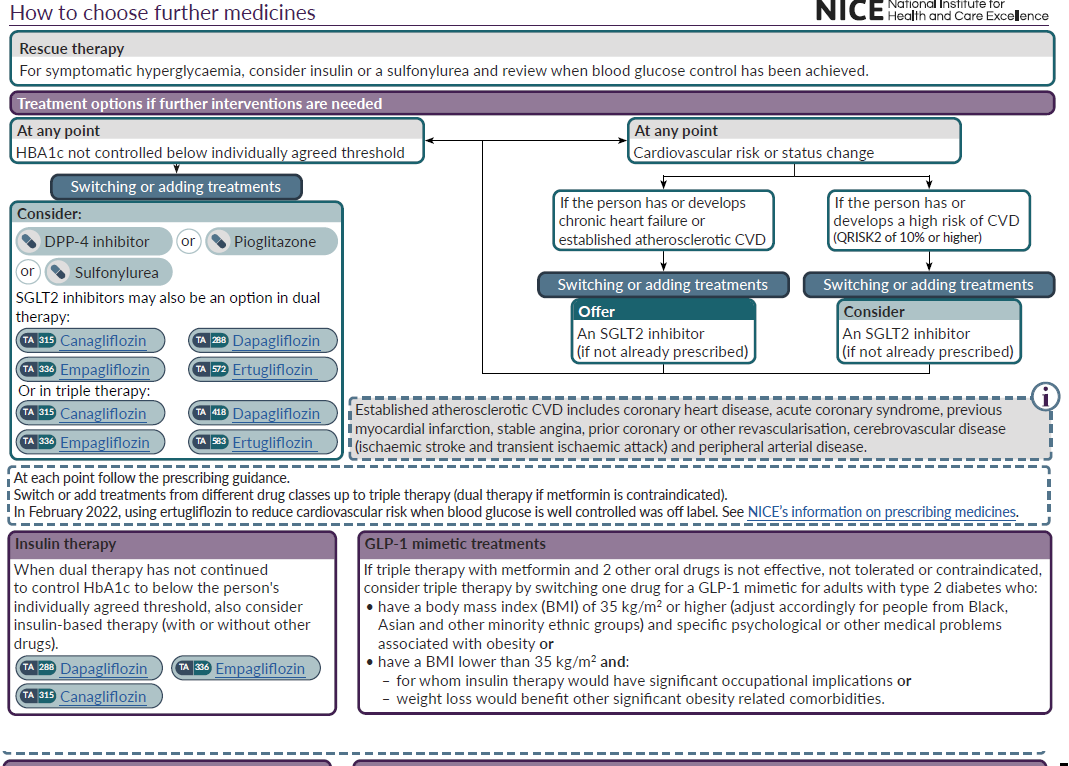
Pioglitazone is an option for adults with type 2 diabetes, if monotherapy has not continued to control HbA1c to below the person's individually agreed threshold for further intervention. Note in some instances that initial therapy will have been a combination of metformin plus an SGLT2 inhibitor (see diagrams below).
Pioglitazone is an option for adults with type 2 diabetes, if dual therapy with metformin and another oral drug has not continued to control HbA1c to below the person's individually agreed threshold for further intervention.
NICE suggest targets for management of type 2 diabetes as (1)
Targets
- for adults with type 2 diabetes managed either by lifestyle and diet, or by lifestyle and diet combined with a single drug not associated with hypoglycaemia, support the person to aim for an HbA1c level of 48 mmol/mol (6.5%)
- for adults on a drug associated with hypoglycaemia, support the person to aim for an HbA1c level of 53mmol/mol (7.0%)
- in adults with type 2 diabetes, if HbA1c levels are not adequately controlled by a single drug and rise to 58 mmol/mol (7.5%) or higher:
- reinforce advice about diet, lifestyle and adherence to drug treatment and
- support the person to aim for an HbA1c level of 53mmol/mol (7.0%)
- and intensify drug treatment
- consider relaxing the target HbA1c level on a case-by-case basis, with particular consideration for people who are older or frail, for adults with type 2 diabetes:
- who are unlikely to achieve longer-term risk-reduction benefits, for example, people with a reduced life expectancy
- for whom tight blood glucose control poses a high risk of the consequences of hypoglycaemia, for example, people who are at risk of falling, people who have impaired awareness of hypoglycaemia, and people who drive or operate machinery as part of their job
- for whom intensive management would not be appropriate, for example, people with significant comorbidities
- if adults with type 2 diabetes achieve an HbA1c level that is lower than their target and they are not experiencing hypoglycaemia, encourage them to maintain it. Be aware that there are other possible reasons for a low HbA1c level, for example, deteriorating renal function or sudden weight loss
HbA1c lower than target:
- If adults with type 2 diabetes achieve an HbA1c level that is lower than their target and they are not experiencing hypoglycaemia, encourage them to maintain it. Be aware that there are other possible reasons for a low HbA1c level, for example, deteriorating renal function or sudden weight loss
Metformin is the usual first line therapy in type 2 diabetes management
- assess the person's cardiovascular status and risk to determine whether they have chronic heart failure or established atherosclerotic cardiovascular disease or are at high risk of developing cardiovascular disease
- based on the cardiovascular risk assessment for the person with type 2 diabetes:
- if they have chronic heart failure or established atherosclerotic cardiovascular disease, offer an SGLT2 inhibitor with proven cardiovascular benefit in addition to metformin
- if they are at high risk of developing cardiovascular disease, consider an SGLT2 inhibitor with proven cardiovascular benefit in addition to metformin
- when starting an adult with type 2 diabetes on dual therapy with metformin and an SGLT2 inhibitor as first-line therapy, introduce the drugs sequentially, starting with metformin and checking tolerability. Start the SGLT2 inhibitor as soon as metformin tolerability is confirmed
- gradually increase the dose of standard-release metformin over several weeks to minimise the risk of gastrointestinal side effects in adults with type 2 diabetes
- if an adult with type 2 diabetes experiences gastrointestinal side effects with standard-release metformin, consider a trial of modified-release metformin
- for first-line drug treatment in adults with type 2 diabetes, if metformin is contraindicated or not tolerated:
- if they have chronic heart failure or established atherosclerotic cardiovascular disease, offer an SGLT2 inhibitor with proven cardiovascular benefit
- if they are at high risk of developing cardiovascular disease, consider an SGLT2 inhibitor with proven cardiovascular benefit
- for first-line drug treatment in adults with type 2 diabetes, if metformin is contraindicated or not tolerated and if they are not in either of the groups in when early introduction of an SGLT2 inhibitor should be considered/offered then:
- a DPP-4 inhibitor or
- pioglitazone or
- a sulfonylurea or
- an SGLT2 inhibitor for people who meet the criteria in NICE's technology appraisal guidance on canagliflozin, dapagliflozin and empagliflozin as monotherapies or ertugliflozin as monotherapy or with metformin for treating type 2 diabetes
- before starting an SGLT2 inhibitor, check whether the person may be at increased risk of diabetic ketoacidosis (DKA), for example if:
- they have had a previous episode of DKA
- they are unwell with intercurrent illness
- they are following a very low carbohydrate or ketogenic diet
- address modifiable risks for DKA before starting an SGLT2 inhibitor. For example, for people who are following a very low carbohydrate or ketogenic diet, they may need to delay treatment until they have changed their diet
- advise adults with type 2 diabetes who are taking an SGLT2 inhibitor about the need to minimise their risk of DKA by not starting a very low carbohydrate or ketogenic diet without discussing it with their healthcare professional, because they may need to suspend SGLT2 inhibitor treatment
For detailed guidance then consult the full guideline (1).
Reference:
Related pages
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page