Assessment of clinical probability of pulmonary embolism (PE)
In patients with suspected PE, a clinical or pre-test probability assessment could be carried out based on the risk factors and clinical features
- it will assess the clinical likelihood of PE in a patient
- the assessment can be carried out either by implicit clinical judgement or by the use of a validated clinical prediction rule e.g.- Canadian rule, by Wells et al or the revised Geneva rule
The Canadian rule by Wells et al divides a person's clinical probability of pulmonary embolism into:
- three-category scheme - low, moderate or high clinical probability
- two-category scheme - PE likely or PE unlikely (2)
The revised Geneva rule also divides the clinical probability of PE in to low, intermediate and high (2). Which ever rule is used the prevalence of PE is about:
- 10% - in patients with a low clinical probability
- 30% - in those with a moderate clinical probability
- 65% - in those with a high clinical probability
Concurrent use of a D-dimer test with the pre test clinical probability score is recommended. e.g. - when a negative d-dimer result is used together with a low or intermediate pre test clinical probability score, it has a 92% sensitivity at excluding pulmonary embolism (3)
A schemata for assessment has been suggested (4)
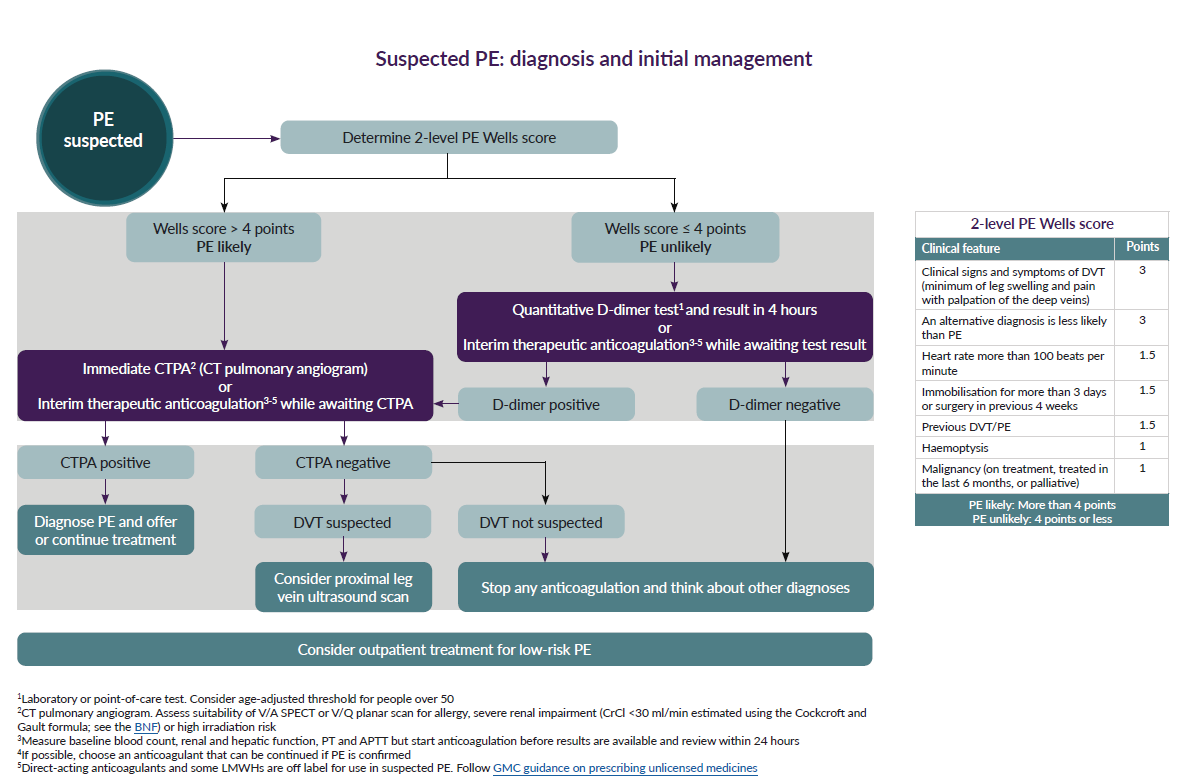
Reference:
- Meyer G et al. Pulmonary embolism. BMJ. 2010;340:c1421
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
- Robinson GV. Pulmonary embolism in hospital practice. BMJ. 2006;332(7534):156-60.
- NICE (March 2020). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing
Related pages
Create an account to add page annotations
Annotations allow you to add information to this page that would be handy to have on hand during a consultation. E.g. a website or number. This information will always show when you visit this page.