Migraine and the combined oral contraceptive pill
Patients should be warned to report increase in headache frequency or onset of focal symptoms - the combined pill should be discontinued and the patient urgently referred if focal neurological symptoms not typical of aura persist for 1 hour or more.
The combined pill is contra-indicated in:
- patients who suffer regular severe migraines lasting more than 72 hours despite treatment
- patients who suffer migraines with typical focal aura
- in patients with migraines without aura if there more than one additional risk factor for arterial disease (1)
- if migraine is treated with ergot derivatives
The combined pill can be prescribed with caution in patients who:
- have migraines without typical focal aura
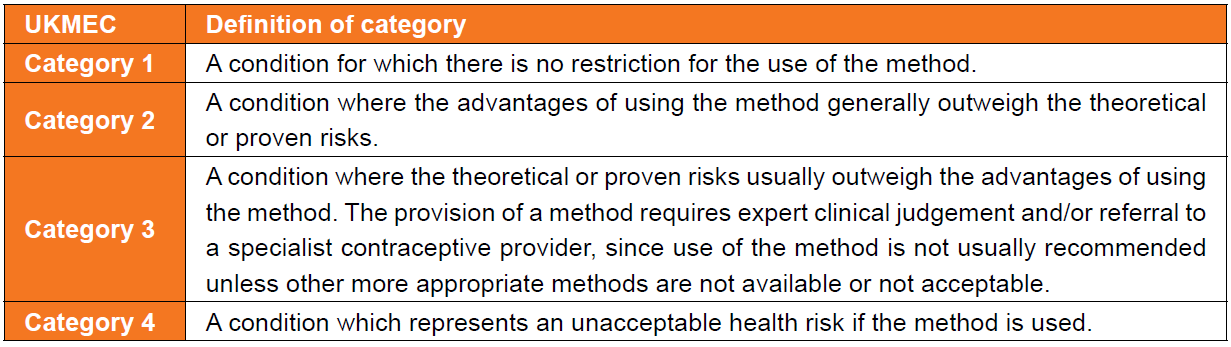
UKMEC Criteria state:
In consideration of UKMEC criteria and combined hormonal contraception (CHC):
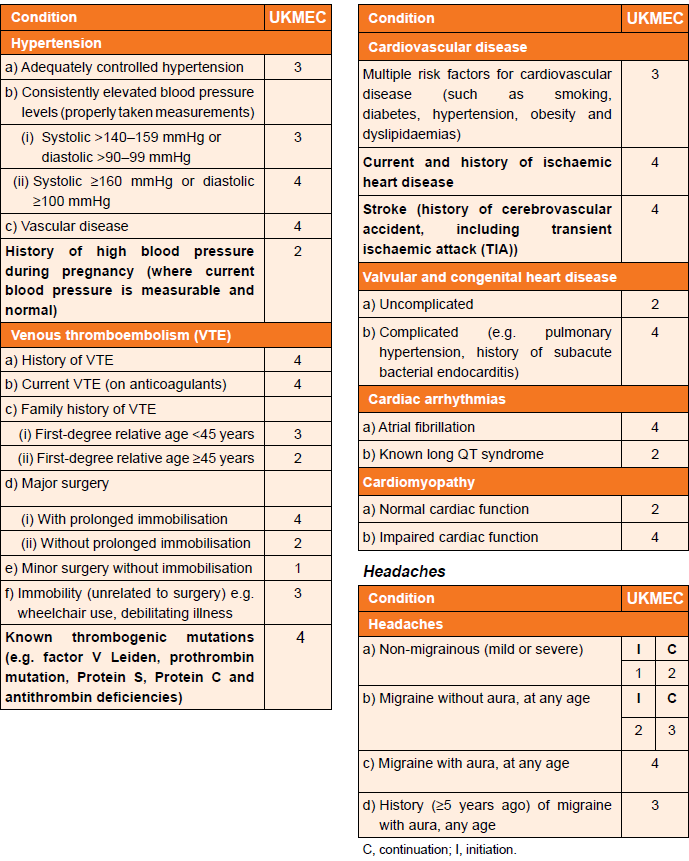
Cardiovascular factors (including migraine) and UKMEC categories (3):
Check the summary of product characteristics before prescribing any combined oral contraceptive pill.
Reference:
- (1) DTB (2000), 38 (1), 1-4.
- (2) BNF 7.3
- (3) FSRH Clinical Guideline: Combined Hormonal Contraception (January 2019, Amended July 2019)
Related pages
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page