Cancer immunotherapy - adverse effects
Side effects
As it stimulates the immune system it can lead to auto immune conditions including colitis, Addison's, hypothyroidism, hyperthyroidism, autoimmune hepatitis and pneumonitis as well as skin conditions. These can present up to a year after treatment has been discontinued.
Side effects stated that are due to immunotherapy (2)
- diarrhoea - colitis
- rash
- auto-immune endocrine disturbance e.g. adrenal insufficiency, hypophysitis, hyper or hypothyroidism
- liver function abnormalities
- neurological side effects e.g. peripheral nerve motor or sensory loss, Guillaine Barre type syndrome, multiple cranial nerve palsies
Immune related adverse events (IrAE) occur in
- up to 90% patients administered ipilimumab
- up to 70% patients administered PD1/PDL1 inhibitors (pembrolizumab, nivolumab, atezolizumab)
First line pembrolizumab (PD1) in lung cancer
- 1 in 10 had a G3 or G4 toxicity (better than chemotherapy)
- 1 in 20 stopped treatment due to toxicity
These are very rarely immunosuppressive drugs
- sick patients are unlikely to have neutropenic sepsis
- mainstay of treatment of immune related adverse events - STEROIDS
Immune Related Adverse Events:
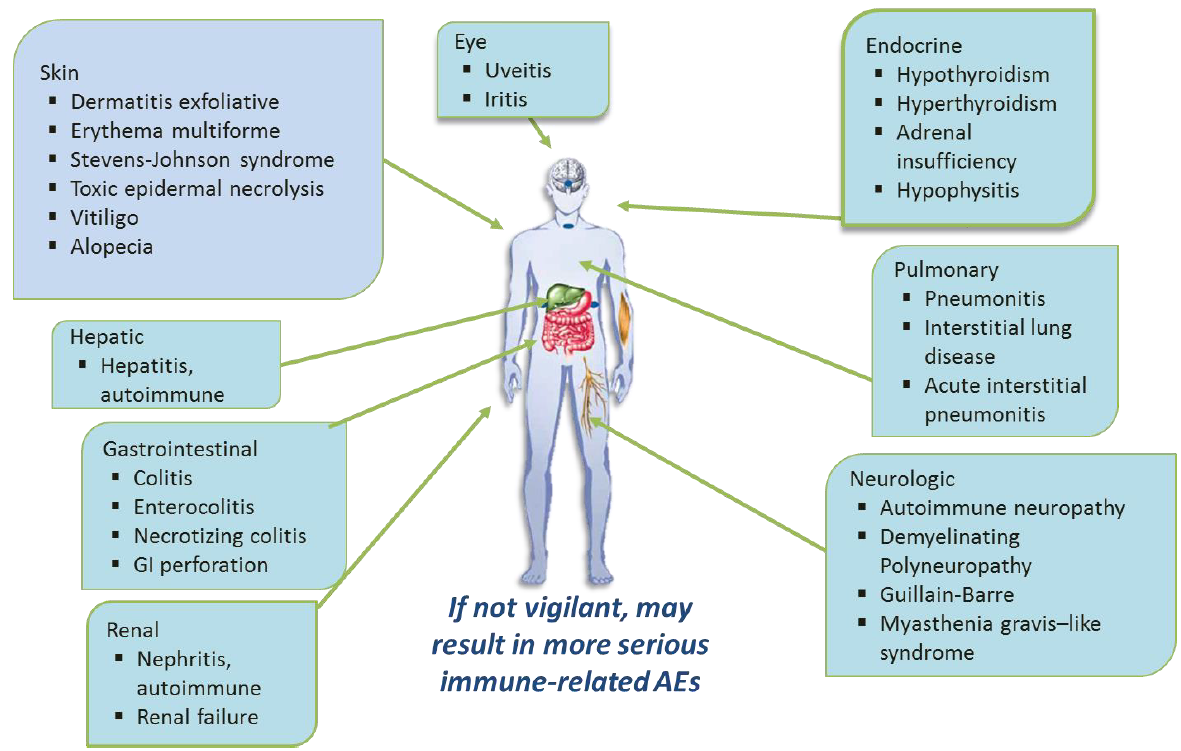
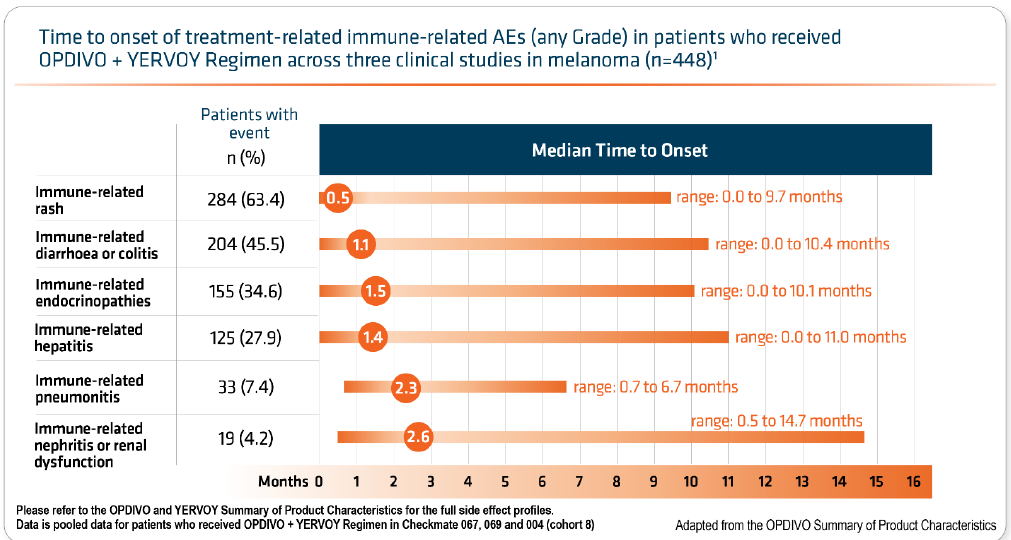
Primary care need to be aware that side effects may not be immediate:
- onset of these toxicities varies but usually starts within the first 8 to 12 weeks of initiation of treatment
Reference:
- Brooks M, Olsson-Brown A. Summary on Immunotherapy for Palliative Care Teams
- The growing impact of cancer immunotherapy. Northern Cancer Alliance Capacity Workshop - For the Delivery of Systemic Anti-Cancer Treatments. Ruth Plummer April 2018
- Haanan J et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2017; 28 (Supplement 4)
Related pages
Create an account to add page annotations
Add information to this page that would be handy to have on hand during a consultation, such as a web address or phone number. This information will always be displayed when you visit this page